BRIDG Overview
BRIDG Stakeholders
The Biomedical Research Integrated Domain Group (BRIDG) Model is a collaborative effort engaging stakeholders from the Clinical Data Interchange Standards Consortium (CDISC), the HL7 BRIDG Work Group, the International Organization for Standardization (ISO), the US National Cancer Institute (NCI), and the US Food and Drug Administration (FDA). The goal of the BRIDG Model is to produce a shared view of the dynamic and static semantics for the domain of basic, pre-clinical, clinical, and translational research and its associated regulatory artifacts.
BRIDG Current Activities
- Providing BRIDG semantics in design of HL7 FHIR research resources/profiles as applicable
Balloting through SDOs
- Latest Ballot: BRIDG 5.3 was balloted through HL7 in Spring 2019 with the following additional content: NCI National Biomedical Imaging Archive (NBIA) semantics, new views, new diagram metadata reports and CDISC SDTM IG v3.2 harmonization.
- Ballot comments were reconciled and model changes published as BRIDG 5.3.1.
- Future Ballots: none currently planned
-
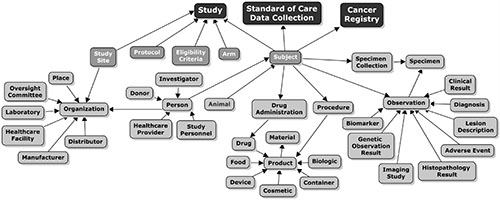 BRIDG High Level Concept Map
BRIDG High Level Concept MapThis concept map provides a simple overview of the range of semantics covered by the BRIDG model.
-
 BRIDG 101
BRIDG 101Download this slide deck for a brief tutorial on the BRIDG model.
-
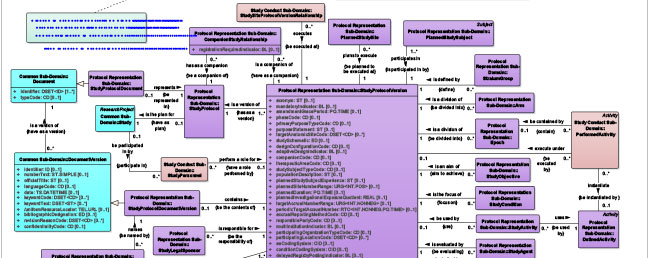 BRIDG Subset Diagrams
BRIDG Subset DiagramsClick here for quick access to a wide range of topic-based UML class diagrams.
